ERBITUX® (cetuximab) Rebranding Pitch
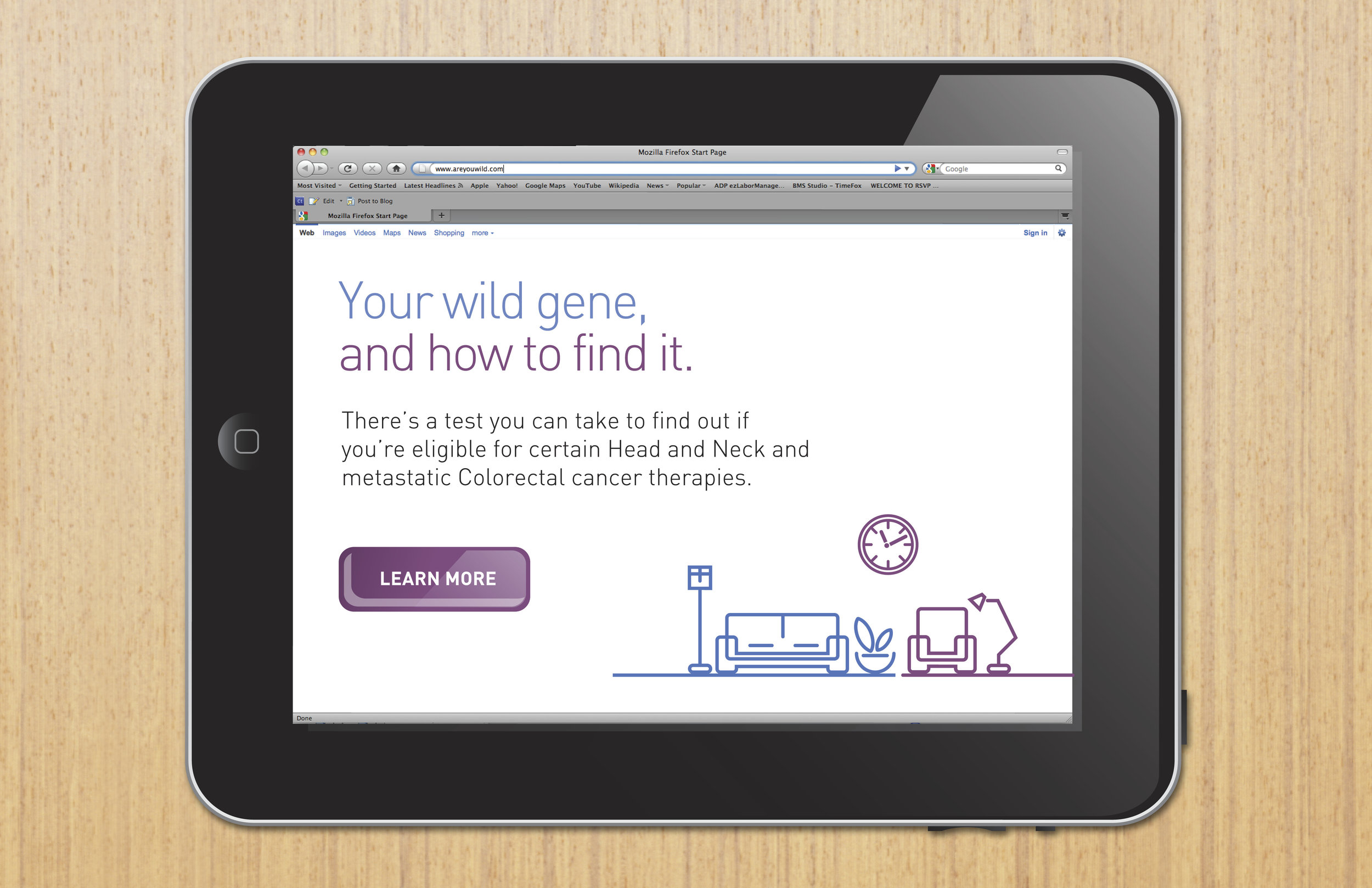
ERBITUX, a Bristol-Myers Squibb Pharmaceuticals drug, is indicated for second-line therapy for head & neck and mCRC cancer. Patients are gene-eligible for therapy after a KRAS test. Only about 50% of doctors actually test after first-line failure; for this reason more patients opt for a drug that does not necessitate a gene test. The goal is to get the doctors to administer the KRAS test earlier so that if/when the patient fails on first-line, they can move right to ERBITUX where appropriate.
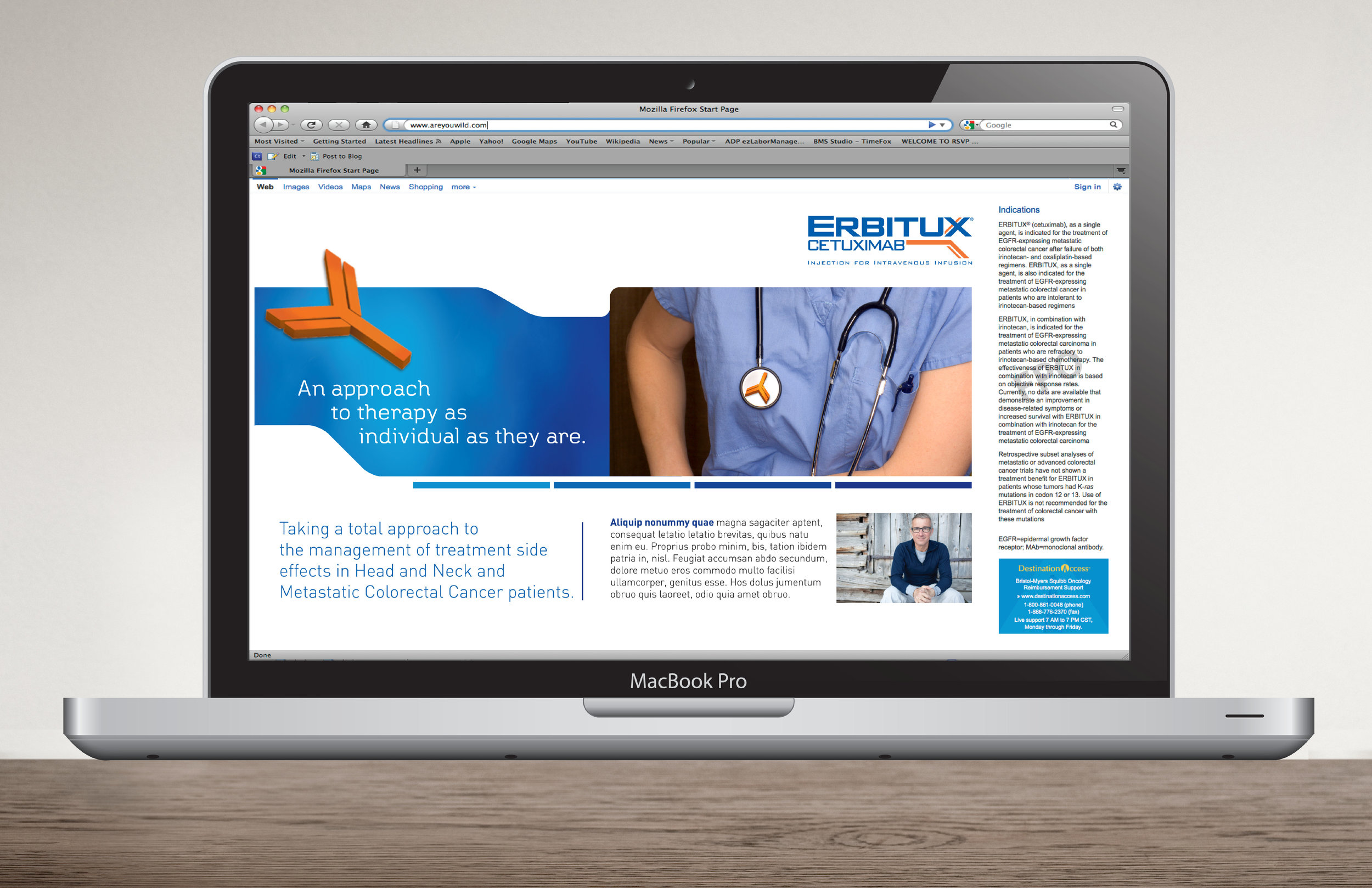
The challenge was to rebrand ERBITUX’s image and copy tone to reflect the BioPharma approach of the Oncology franchise known as Immuno-Oncology (cancer treatment utilizing the body’s own immune system).
Recent market testing also showed that the ERBITUX brand was perceived as visually cold and distant to HCPs and consumers. Our campaign then focused on a Personalized Medicine approach: Use of KRAS testing provides health professionals an additional measure of “personalized” information that allows them to feel more validated in their choice of therapy.
My core responsibilities were to develop the core creative & messaging, layout and design of all components associated with this rebranding pitch.






Unbranded communication development opportunity
The proactive testing of patient KRAS biomarkers is an important educational element for the prescribing of ERBITUX. We also developed unbranded communications for immediate second-line usage with an educational campaign that focuses on the value of biologic biomarkers and early KRAS testing.